The US Food and Drug Administration (FDA) has approved the PulseSelect Pulsed Field Ablation (PFA) System (Medtronic) for the treatment of both paroxysmal and persistent atrial fibrillation (AF), the manufacturer has announced.

The technology uses microsecond-scale pulsed electrical fields for pulmonary vein isolation (PVI) to reduce AF and is the first PFA system to receive FDA approval for the condition. Medtronic received a CE mark for the system in the European Union in November.
Because PFA technology uses a largely nonthermal mechanism of cell death, risk to surrounding tissues is lower than with conventional catheter-based thermal ablation, which isolates pulmonary veins by heating or freezing the tissue, Medtronic notes in the announcement.
FDA approval of PulseSelect was based on results from the multicenter nonrandomized PULSED AF trial, a study of 300 people with recurrent, symptomatic paroxysmal AF or persistent AF.
As reported previously by theheart.org | Medscape Cardiology, the trial showed a 0.7% safety event rate and clinical success rates of 80% both in patients with paroxysmal and with persistent AF.
After a 90-day postprocedure blanking period, 66.2% of patients with paroxysmal AF and 55.1% of those with persistent AF met the primary efficacy endpoint of freedom from acute procedural failure, AF recurrence, repeat ablation, direct-current cardioversion, left atrial surgery, or escalation of antiarrhythmic drugs.
At 1 year, 70% of the paroxysmal-AF group and 62% of the persistent-AF group remained free of any recurrent atrial arrhythmia; 80% and 81%, respectively, met the clinical-success endpoint of freedom from symptomatic atrial arrhythmia recurrence.
Investigators say PFA creates the lesions by exposing the myocardial targets to high-gradient electric fields, leading to a nonthermal electroporation process that rapidly renders myocyte membranes "hyperpermeable," inducing apoptotic cell death.
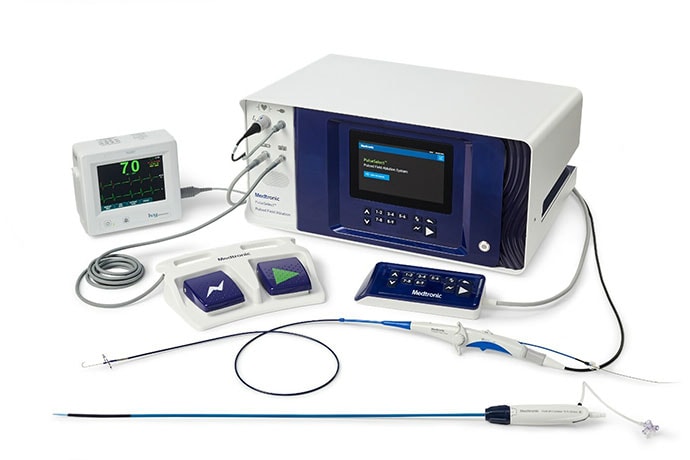
Although PFA ablation energy differs from the heat of RF ablation or cryoablation's deep freeze, investigators note that the catheters are similar to those used in the past.
"In my clinical experience with the catheter, it was designed for AF ablation procedures," trial investigator Amin Al-Ahmad, MD, clinical cardiac electrophysiologist at St. David's Medical Center in Austin, Texas, said in a press release. "The learning curve in using the catheter and system is short, and the catheter enables the operator to deliver fast and controlled pulsed field energy for AF ablation."
Results from the noninferiority ADVENT trial presented at the European Society of Cardiology in August showed that Boston Scientific's PFA system achieved similar results to conventional thermal ablation in a head-to-head comparison.
The company says commercialization of the PulseSelect PFA system will begin in early 2024.
Kelli Whitlock Burton is a reporter for Medscape Medical News covering neurology and psychiatry.



Comments