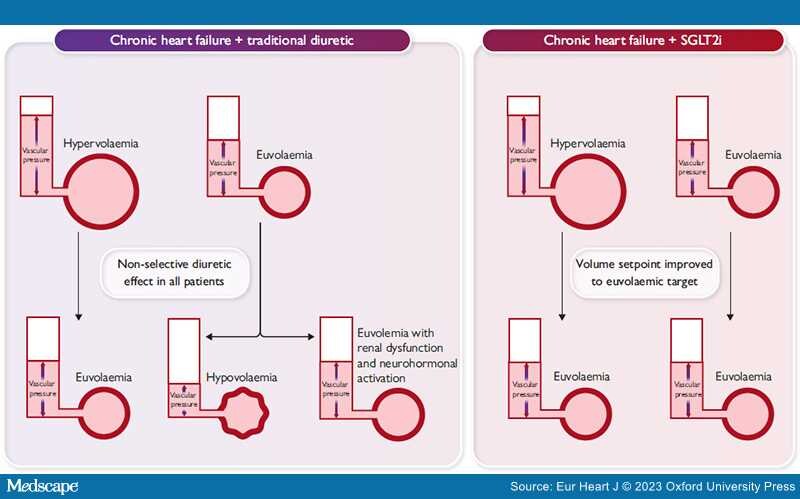
Graphical Abstract
Non-selective diuretic use in patients with heart failure succeeds in treating patients with true hypervolemia, but frequently leads to hypovolemia or excessive renal dysfunction and neurhormonal activation when administered to patients who were euvolemic at the time of administration. Administration of SGLT2 inhibitors (SGLT2i) may effectively improve the volume setpoint for the kidneys, allowing for volume excretion in the setting of hypervolemia, but allowing for counterregulatory mechanisms to preserve volume when euvolaemia is present.
After years of treatment decisions based upon expert consensus alone, patients with heart failure (HF) and preserved ejection fraction (HFpEF) finally have a robust, evidence-based treatment in the sodium–glucose co-transporter 2 (SGLT2) inhibitors.[1] The SGLT2 inhibitors dapagliflozin and empagliflozin have been shown to reduce the composite of HF hospitalization and cardiovascular death, improve patient-reported health status, enhance functional capacity, and delay the age-related reduction in kidney function in patients with HFpEF and HF with mildly reduced EF (HFmrEF).[2–4] However, the mechanisms responsible for these salutary effects remain unclear. Although data vary across studies, SGLT2 inhibitors appear to reduce plasma volume and body weight, promote natriuresis and diuresis, improve intraglomerular haemodynamics, stimulate erythropoiesis, and enhance nutrient deprivation signalling pathways, and may also improve myocardial structure and function.[5] Notably, the increase in haematocrit has been found to be a primary mediator of the benefit of SGLT2 inhibitors on hard outcomes, which may be a reflection of the erythropoietic and diuretic effects of these drugs.[6–8]
Loop diuretics, on the other hand, are more of a necessary evil in HF. They reduce pulmonary and systemic congestion and improve symptoms, but cause neurohormonal activation, kidney dysfunction, and electrolyte abnormalities, and worsen sodium avidity,[9] making it preferable to avoid these agents if possible, or use the lowest doses needed to maintain euvolaemia. Unlike loop diuretics, SGLT2 inhibitors work in the proximal tubule and may potentiate the efficacy of loop diuretics.[10] In the DAPA-HF trial, patients with HF and reduced EF (HFrEF) treated with dapagliflozin were more likely to have a down-titration in diuretic dose, and less likely to require an escalation in diuretic dose compared with those treated with placebo, but mean diuretic dose did not differ between groups.[11] In the EMPEROR-Preserved trial, patients with HFpEF/HFmrEF treated with empagliflozin were 27% less likely to require diuretic intensification compared with those treated with placebo, but effects on mean diuretic dose were not reported. Paradoxically, despite these consistent signals for a loop diuretic-sparing effect, signals for diuresis-related adverse events were consistently absent across the trials, with rates of acute kidney injury improved, and no significant worsening of volume depletion events.
In this issue of the European Heart Journal, Chatur and colleagues report results from a pre-specified analysis of the DELIVER trial examining the impact of baseline diuretic use on dapagliflozin efficacy, as well as any longitudinal changes in diuretic use on treatment.[12] Reduction in the risk of the primary endpoint and safety data were consistent across doses and types of diuretics, with no significant interaction. In agreement with the analyses from EMPEROR-Preserved, treatment with dapagliflozin reduced new initiation of diuretics [hazard ratio (HR) 0.68; 95% confidence interval (CI) 0.55–0.84], but there was no effect on discontinuation, perhaps driven in part by inertia in clinical decision-making but also by the masterful renal adaptation of the kidney to diuretics (see below). Loop diuretic dose increase was less frequent, dose decrease was more frequent, and the longitudinal increase in loop diuretic dose was significantly attenuated with dapagliflozin. Importantly, safety was also similar across the spectrum of diuretic use, with no excess risk of renal adverse events or events that might suggest volume depletion.[12]
Normal kidneys filter plasma volume >30 times per day, resulting in well over 100 L of water and 1 kg of salt reabsorbed each day. Approximately 70% of this reabsorption occurs in the proximal tubule, with a progressively smaller amount of reabsorption occurring while traversing the nephron distally. SGLT2 inhibitors have significant proximal tubular effects by both blocking sodium–glucose co-transport and modulating sodium reabsorption through the sodium hydrogen exchanger 3, which drives the largest proportion of sodium reabsorption in the nephron. It is this combined effect that probably explains why SGLT2 inhibitors can block >20% of proximal tubular reabsorption.[13,14] In light of these powerful proximal tubular effects, it is intuitive how the signal for lower requirement for loop diuretic therapy would emerge from the SGLT2 inhibitor HF trials.
However, it is not intuitive how such a powerful proximal tubular effect translates into such a modest observed effect on diuretics. A 20% reduction in proximal tubular water/salt reabsorption should not only be able to entirely replace loop diuretics, but should also induce a universally lethal volume depletion within days of starting SGLT2 inhibitor treatment. Obviously, this does not occur, due to rapid renal compensation. Although the proximal tubular effects of SGLT2 inhibitors seem to persist long term, there is a prompt increase in sodium reabsorption in distal nephron segments. Importantly, this phenomenon is not unique to the SGLT2 inhibitors—loop diuretics should also be capable of inducing a rapidly lethal diuresis with their ability to block ~20% of sodium reabsorption at a more distal segment than SGLT2 inhibitors, but renal compensation again intervenes, preventing overdiuresis in most circumstances. In the end, the nephron is quite nimble, with all existing diuretic agents blocking sodium reabsorption in one segment ultimately leading to an equivalent increase in sodium reabsorption in another segment. Put another way, every patient that is on a stable dose of loop diuretic has developed a degree of diuretic resistance that keeps them at that stable volume status. It is only through this adaptive compensation that diuretics are safe with a usable therapeutic window. However, this adaptation to diuretics often comes at the cost of worsening kidney function and neurohormonal activation.
So, what is it about SGLT2 inhibitors that allow a population-level improvement in volume status, without the seemly obligatory diuretic-related adverse effects such as worsening kidney function, electrolyte abnormalities, neurohormonal activation, hyperuricaemia, and volume depletion events? For example, we would expect indiscriminate addition of a thiazide diuretic to euvolaemic patients on a loop diuretic (as we do with SGLT2 inhibitors) to cause these exact adverse events. However, the expansive SGLT2 trial experience documents that this does not occur, and in many studies we paradoxically observe reduction in adverse events such as acute kidney injury, electrolyte abnormalities, and improvement in uric acid levels.
The proximal tubular location of SGLT2 probably drives the paradox observed in trials such as DELIVER, where a diuretic/diuretic-sparing effect is seen, without the downsides of a drug that induces a diuretic/diuretic-sparing effect. It is as if the drug is working by making the kidney operate more 'normally' in handling sodium, rather than the 'brute-force' addition of a second diuretic such as a thiazide. Blocking a large amount of sodium reabsorption in the proximal nephron probably provides this opportunity by (i) increasing delivery of salt-rich fluid to salt-sensing elements spanning the entire remainder of the nephron, and (ii) having the entire remainder of the nephron available to compensate and reabsorb this salty fluid (if required). For example, enhanced sodium chloride delivery to the macula densa results in a relative suppression of renin–angiotensin–aldosterone system activation and other compensatory mechanisms, but this salt and fluid then traverse the remaining nephron segments to undergo compensatory reabsorption as needed, avoiding overdiuresis.
In light of this physiology, SGLT2 inhibitors probably should be conceptualized not as diuretics but rather as modulators of the volume set point (Graphical Abstract). This vantage point allows us to reconcile the lack of excess risk of volume depletion reported with these agents with the observation of improved volume status and reduction in volume overload-associated events such as HF hospitalization. Healthy people have a normal volume set point, and they too enjoy an absence of hypervolaemic and hypovolaemic events since the kidneys expertly maintain them at this normal volume. The state of HF, regardless of EF, deceives the kidneys into 'believing' that the patient is below their volume set point and thus it is in their best interest to retain salt and water. If SGLT2 inhibitors can in fact recalibrate the volume set point and mitigate this deception, that would certainly be concordant with the observed remarkable reduction in HF hospitalizations seen so early after initiation of treatment. Furthermore, the observation that SGLT2 inhibitor benefit does not differ by baseline loop diuretic dose is also concordant with a recalibration of the set point rather than a simple additive diuretic effect, since any patient at risk for a future volume-related HF hospitalization should benefit from improvement in the volume set point, regardless of their baseline diuretic status. The elegant analysis from Chatur et al. in the present issue,[12] in tandem with prior trials and mechanistic studies, is most consistent with this mechanism and helps us inch closer to understanding how these remarkable drugs reduce the risk of adverse outcome in patients with HFpEF, HFmrEF, and HFrEF.
Acknowledgements
B.A.B. is supported in part by research grants from the NIH (R01 HL128526 and U01 HL160226) and the United States Department of Defense (W81XWH2210245). J.M.T. is supported in part by NIH R01HL139629, R01HL128973, R01HL148354, R01DK130997, and R01DK130870.
Data availability
No new data were generated or analysed in support of this research.
Funding
All authors declare no funding for this contribution.
Eur Heart J. 2023;44(31):2944-2946. © 2023 Oxford University Press
Copyright 2007 European Society of Cardiology. Published by Oxford University Press. All rights reserved.