Abstract and Introduction
Abstract
Purpose of Review: Immunotherapy, a treatment modality currently synonymous with immune checkpoint blockade remains a challenge for prostate cancer. Despite multiple phase 3 trials using checkpoint inhibitors in combinatorial approaches, there have been no benefits to date in overall survival or radiographic progression free survival. However, newer strategies prevail that are directed to a variety of unique cell surface antigens. These strategies include unique vaccines, chimeric antigen receptor (CAR) T, bispecific T cell engager platforms, and antibody-drug conjugates.
Recent Findings: New antigens are being targeted by various immunologic strategies. These antigens are pan-carcinoma as they may be expressed on a variety of cancers but remains effective targets for therapeutic attack.
Summary: Immunotherapy with checkpoint inhibitors alone or in combination with a variety of agents such as chemotherapy, poly-ADP ribose polymerase (PARP) inhibitors or novel biologics have met with failure in the endpoints of overall survival (OS) and radiographic progresson-free survival (rPFS). Despite these efforts, other immunologic efforts to develop unique tumor-targeted strategies should be continued.
Introduction
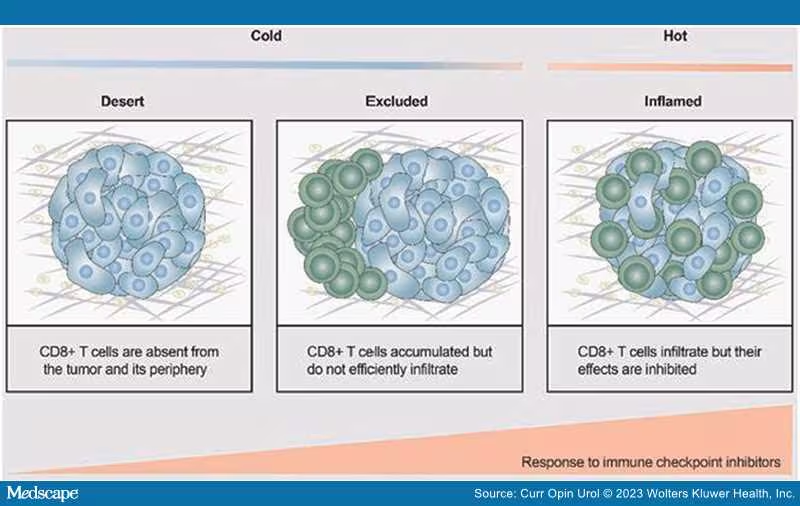
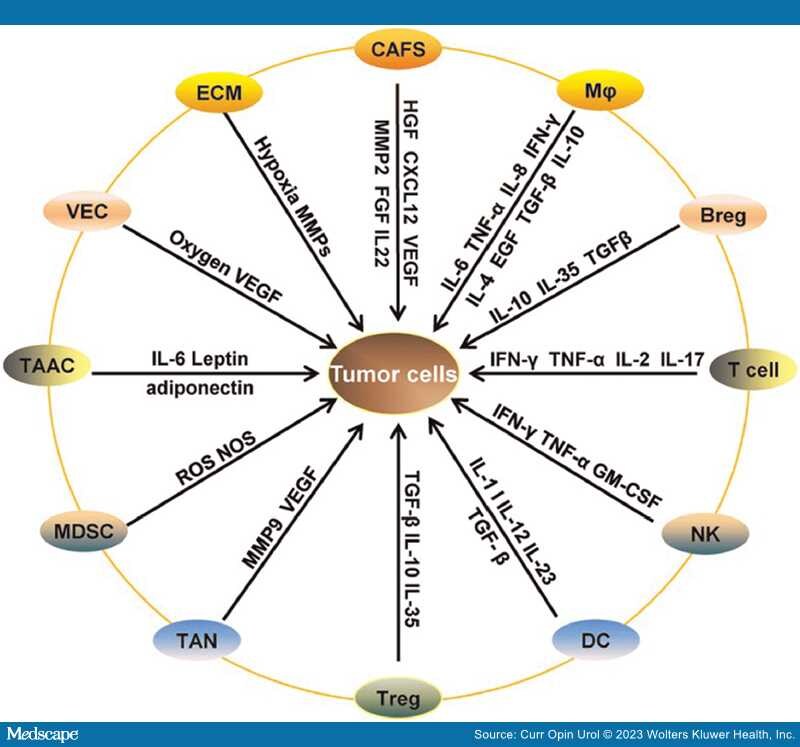
Treatments for both de novo metastatic and metastatic castration resistant prostate cancer patients have grown considerably both in number and by class, the latter including the addition of poly-ADP ribose polymerase (PARP) inhibitors and checkpoint inhibitors. No longer does a treatment become a "one size fits all"; patient subsets are routinely being identified in order to develop a "profile" of the biologic behavior of the tumor, thereby leading to better treatment with more target-directed agents. The successes to date in prostate cancer are great; at least seven different approaches to treat newly diagnosed de novo metastatic disease. This is an unheralded feat for a disease that did not even have a standard of care chemotherapy until docetaxel was approved in 2004. But despite our efforts, immune based therapies have not managed to meet the pinnacle of success as with other treatments. Understanding the immune microenvironment in prostate cancer is considered of paramount importance to developing immune targeted agents for this disease. Despite efforts that have revealed an underlying immunologic desert-like or bland internal environment devoid of a rich "oasis" of immune cells, attempts to transform this "cold" into a "hot" responsive environment have not yielded robust antitumor effects[1,2] (Figure 1). Clearly, there are other mechanisms at play that may contribute to the lack of response to a variety of immunologic platforms (Figure 2). Most of the research in immune agents have shown success in preclinical trials; these have not panned out when translated into the clinical arena. There have been fewer efforts to understand why only a rare few respond, and more importantly, why the majority do not. The only glimmer of a potential success was with the approval of Sipuleucel-T,[3] although despite being the first immune therapy for a solid tumor, that is, prostate cancer, to provide a survival benefit, it is not robustly embraced as a frequently used treatment. In many cases, it is used as a temporizing agent in the metastatic castration-resistant prostate cancer (mCRPC) patients who are asymptomatic or minimally symptomatic with the idea that this drug is somehow changing the immune constitution locally and systemically and therefore may modulate future treatment responses. While there are no hard data to support or deny this rationale, nevertheless, real world studies, although retrospective, suggest a benefit in diverse populations[4]
Figure 1.
Conceptual view as to the difficulties in getting immune cells into the tumor milieu. Reproduced courtesy of open access of Ivyspring International Publisher [1].
Figure 2.
The complex interplay of factors within the tumor leading to a hostile microenvironment. Reproduced courtesy of open access of Springer Nature [2].
Why Does Prostate Cancer Continue to be a Treatment Challenge?
At first glance, one could make the argument that it is a bone tropic disease, hence a packed bone marrow and increased stroma may drive out immune effector cells, or there may be inhibitory factors within the marrow such as cytokines or adenosine that may foster an adverse environment. Another concern is that we may not be selecting the right patient populations and should consider patients with lesser tumor burdens, that is, either newly diagnosed patients where direct impact on the tumor may be feasible either in the neoadjuvant or later in the biochemically relapsed state, respectively. Despite efforts with directed therapies in these groups, no immunologic platform has demonstrated clinical benefit although immune responses as indicated by high antibody titers to the immunogen or changes in T cell subsets were noted.[5,6]
Can Success in the Phase II Setting Lead to Failure in the Phase III Setting?
There was considerable enthusiasm for PROSTVAC, a unique immunotherapeutic platform that used a heterologous prime boost strategy to elicit durable immune responses. It utilized two recombinant pox viruses along with a PSA transgene and the incorporation of three co-stimulatory molecules including LFA-3, B7.1 and iCAM (TRICOM).[7,8] The strategy used a priming dose of the vaccinia virus that encoded the PSA-TRICOM followed by 6 subsequent injectors or "boosters" dose of fowlpox that contained the same co-stimulatory molecules. PSA-specific T cell responses were obtained and the early phase II data of 125 patients with metastatic castration resistant prostate cancer suggested a survival benefit. This led to a large international phase III validation trial "PROSPECT"[8] which did not support the observations of the phase 2 trial and was stopped for medical futility.
Pursuing Checkpoint Inhibitors in an Immunologically Insensitive Disease.
Despite the lack of efficacy of checkpoint inhibitors in mCRPC, efforts have been ongoing to use combinatorial approaches of checkpoint inhibitors with standard of care chemotherapies or other biologic agents in an attempt to demonstrate either additive or synergistic antitumor effects. Four phase 3 pivotal trials, the "KEYNOTE" series, investigated combinations of pembrolizumab with a variety of agents. The impetus for these combinations was based on early but encouraging data from the phase 1b/2 KEYNOTE 365 trial that combined pembrolizumab with docetaxel and prednisone in patients with mCRPC who were chemotherapy-naïve and/or failed abiraterone or enzalutamide with progressive disease.[9] Docetaxel was given at the standard dose of 75 mg/m2 every 3 weeks with prednisone 5 mg po twice daily and pembrolizumab at 200 mg iv every 3 weeks. Antitumor activity was observed: PSA response rate was 34% and overall response rate (ORR) was 23%. Median radiographic progresson-free survival (rPFS) and overall survival (OS) were 8.5months and 20.2 months, respectively. Not unexpectedly, there were treatment-associated adverse events. The KEYLYNK-010 study[10] examined the combination of pembrolizumab plus the PARP inhibitor Olaparib. This robust 793 patient trial randomized molecularly unselected patients with mCRPC 2:1 to receive standard every 3 week pembrolizumab plus 100 mg orally of Olaparib twice daily and either standard dosed abiraterone with prednisone or enzalutamide 160 mg po daily. The addition of either androgen receptor signaling inhibitor (ARSI) was based on which of the two the patient had received previously. Though the primary endpoints were rPFS and OS, the Sponsor elected to discontinue the trial based on input from the Data and Safety Monitoring Committee.[11] This trial was followed by KEYNOTE-991,[12] a phase 3 randomized, double-blind placebo-controlled multicenter trial of 1251 patients that examined the combination of pembrolizumab with enzalutamide in patients with metastatic hormone sensitive prostate cancer. Patients were naïve to ARSIs and were further stratified regarding prior treatment with docetaxel based on high tumor volume. Patients were randomized 1:1 with standard doses and schedules of these drugs. The rationale for using enzalutamide was based on preclinical studies that suggested that enzalutamide may have an immunomodulatory effect; thus when used in combination with a checkpoint inhibit may synergize for added efficacy. While a reasonable consideration, this trial was also halted prematurely due to lack of efficacy.[13] There were concerns about a higher incidence of grade 3–5 adverse events. Lastly is the KEYNOTE-921, a randomized, double-blind phase 3 study that examined the safety and efficacy of pembrolizumab with docetaxel in patients with mCRPC who were previously treated with an ARSI. This phase 3 trial enrolled 1030 patients to either the combination or docetaxel alone with the primary dual endpoints of OS and rPFS.[14] Patients on the combination arm received a median of 12 cycles [range 1–35] of pembrolizumab and nine cycles of docetaxel [range 1–12]. Those on the placebo plus docetaxel arm received a median of 12 cycles of placebo [range 1–35] with nine [range 1–10] cycles of docetaxel. Median rPFS was 8.6 months compared with 8.3 months with placebo plus docetaxel. Median OS was 19.6 vs. 19 months, respectively [P = 0.1677, HR = 0.92]. No new safety signals or serious adverse events were noted.[14]
Several additional studies have been reported; one with nivolumab and rucaparib suggested activity in patients with homologous repair deficiency (HRD) who are either post chemotherapy or have chemotherapy-naïve mCRPC with particular activity noted in patients with BRCA1/2 mutations.[15] Patients with either HRD or BRCA+ disease had ORR of 17.2% and 33.3%, respectively with an ORR for all treated patients of 10.3%. Median OS was 20.2 months for all patients treated; the HRD group was 22.7 months and 20.2 months in the BRCA cohort, albeit the number of patients per cohort were not identical. There were no new safety signals. Another trial examined in the phase 2 setting, the combination of durvalumab, a human Igg1-k monoclonal antibody against programmed cell death ligand 1 (PDL1) with Olaparib in patients with mCRPC both with and without germline or somatic DNA damage repair (DDR) mutations.[16] These patients were permitted to have had prior ARSIs and received standard dose olaparib 300 mg po bid with durvalumab 1500 mg iv every 28 days. This study was predicated on the observations of potential synergy between PARP inhibition with immunotherapy and that the DNA damage and hence potentially actionable neoantigens and mutations incurred by the PARP inhibitor could lead to an increased production of interferon-ϒ via the Stimulator of Interferon Genes intracellular pathway. Several interesting observations were noted. This included a correlation between the baseline fraction of myeloid-derived suppressor cells and treatment response. Also observed were immune responses that were associated with benefit. This included of a variety of markers including CD83 on CD141+mDCs leading to a prolonged PFS. Among the nine responders, PSA decline of 85% was reported with duration of response of 16.1 months particularly in those patients harboring germline mutations in DDR genes. The 12 month PFS was 51.5% of which more than 50% were taxane-resistant. Though a small study, it provided insight into the immunomodulatory impact of these two agents and provides some further understanding that the immune system can be reflective of a change in the overall biology of the disease, an observation that has not been consistent in multiple trials.[16]
Despite the lack of disease impact by checkpoint inhibitors either alone or in combination with chemotherapy or biologic agents, it remains unclear as to the rationale for the plethora of ongoing trials that continue to examine newer subclasses of these checkpoint inhibitor either alone or in combination. However, there still are patients who continue to benefit from checkpoint inhibitors who do not demonstrate favorable response features such as MSIhi but nevertheless derive benefit. As was seen in earlier phase I/II trials with the anti-CTA-4 monoclonal antibody ipilimumab, there were patients at the tail of the curve who had durable responses albeit for unclear reasons. The original phase I/II trial of ipilimumab suggested that there was a signal of benefit in patients with mCRPC who had received up to three sites of radiation to disease sites, followed one day later by ipilimumab.[17] The radiation was performed to elicit antigen release followed by the antibody in an attempt at an abscopal effect. Given reasonable tolerability and a suggestion of benefit, this trial provided the rationale for the phase 3 trial by Kwon et al.[18] in patients with mCRPC. There was no significant difference in OS in those patients who were treated with ipilimumab or placebo, respectively. Another multicenter, double-blind phase 3 trial in the mCRPC state by Beer et al.[19] was performed in asymptomatic or minimally symptomatic patients who were chemotherapy naïve; there was no OS benefit, although once again there was a subset of patients at the tail end of the Kaplan-Meier curves who had durable responses and increased PFS.
Is Immunotherapy no Longer Appropriate for Prostate Cancer?
The answer is a definite NO. Colleagues assume that "immunotherapy" is tantamount to checkpoint inhibition. However, immunotherapy is much more expansive than the checkpoint inhibitors. Immunotherapy also included peptide and DNA vaccines, all of which had limited success in phase 1 or 2 trials, respectively. In many instances, high titers of immunoglobulin M and immunoglobulin G antibodies against the immunogen were elicited, however there was no impact on PSA or the biology of the cancer. New and noteworthy are antibody-drug conjugates that can target pan-carcinoma molecules that are not uniquely expressed on the prostate cancer cell surface, such as Prostate Specific Membrane Antigen (PSMA),[20,21] Trophoblast cell surface antigen 2,[22] Six Transmembrane Epithelial Antigen of the Prostate (STEAP1),[23,24] CD46 and B7/H3.[25] Cellular therapy is a robust area of investigation. New and noteworthy are antibody-drug conjugates that can target molecules that are expressed on the prostate cancer surface, such as PSMA[20] or B7/H3.[25,26] Also evolving is the multigenerations of bi-specific T cell engagers (BiTES) directed against STEAP1[27] or PSMA.[28,29] BiTES constructs have been evolving with greater specificity and durability at the tumor site. They are now being targeted with moderate success to Prostate Stem Cell Antigen (PSCA), cluster of differentiation 134 - CD134, cluster of differentiation 3 and Delta-Like Ligand 3.
Chimeric antigen receptor (CAR) T cells directed to STEAP-1[30] or PSCA[31] have shown promise as have CAR T cells for PSMA.[32–34] A PSMA CAR T cell based on a novel transposon platform has been reported along with normalization of post vs. pre FDG and PSMA PET scan.[34] PSMA CAR T cells have also been isolated from the bone of such patients indicating that despite the increased stroma and packed marrow often seen in patients with mCRPC, CAR T cells can make their way into the bone marrow and effect antitumor responses that can last ~one year.[34]
Curr Opin Urol. 2023;33(5):390-395. © 2023 Wolters Kluwer Health, Inc.