Abstract and Introduction
Abstract
Background and Objective: Biliary tract cancers (BTCs), including cholangiocarcinoma and gallbladder cancer, are a relatively rare group of cancers with a poor prognosis. Over the past decade, the utilization of next-generation sequencing has led to the identification of multiple actionable somatic aberrations in BTCs. Subsequently, new therapies have been created to target these molecular alterations and have been incorporated into clinical practice. In this review, we outline therapies that have been previously studied, and those that are under investigation, to target genomic alterations with the goal of improving survival in patients with advanced disease.
Methods: A literature search was performed to identify phase I, II, and III trials of targeted therapies in patients with advanced BTCs published between January 1, 2010 and October 1, 2022. Medline (via PubMed) and ClinicalTrials.gov were searched for relevant studies and 415 trials were identified. The search strategy was performed using keywords including: biliary tract cancer, cholangiocarcinoma, gallbladder cancer, chemotherapy, targeted therapy, randomized trials, controlled trials, phase I, phase II, and phase III. Search results were imported into EndNote X 9.1.
Key Content and Findings: Overall, immune checkpoint inhibitors, fibroblast growth factor receptor (FGFR) inhibitors, isocitrate dehydrogenase (IDH) inhibitors, and human epidermal growth factor receptor 2 (HER2)-directed therapies have all shown promising results with regard to efficacy in patients with advanced BTCs studied in clinical trials. A number of other agents have also been studied in early-phase trials.
Conclusions: Targeted agents can improve survival in patients with advanced BTCs and have substantially increased the number of potential therapeutic options in patients with refractory disease. The therapeutic landscape of targeted therapies for patients with advanced BTCs continues to evolve based on improvements in detection of genomic alterations.
Introduction
Intrahepatic cholangiocarcinoma (IC), distal extrahepatic cholangiocarcinoma (EC), hilar (Klatskin) cholangiocarcinoma, and gallbladder cancer, collectively known as biliary tract cancers (BTCs) arise from the epithelium of the biliary tree and are relatively rare cancers. The global incidence of BTCs is estimated to be between 0.3 to 6 per 100,000 people, with the highest incidence in southeast Asia (age-standardized rate as high as 3.00 in South Korea) and lowest in Western countries (age-standardized rate as low as 0.66 in the United Kingdom).[1,2] In the United States, the incidence of cholangiocarcinoma, particularly IC, is rising.[3,4] Risk factors for BTCs include liver fluke infection, primary sclerosing cholangitis, hepatolithiasis, choledochal cysts, alcohol consumption, tobacco use, hepatitis B and hepatitis C infection, and metabolic conditions such as obesity, diabetes, and nonalcoholic fatty liver disease.[5–9] Although some progress has been made with respect to improving the survival of patients with BTCs over the past decade, historically, these malignancies are associated with a poor prognosis, with 5-year overall survival (OS) rates of less than 15%.[10–12]
Early detection of BTCs greatly impacts prognosis. Currently, no screening guidelines exist or are recommended for BTCs. When BTC is suspected, contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) or computed tomography (CT) are the imaging modalities of choice to evaluate for vascular anomalies, satellite lesions, lymph node involvement, and metastatic disease.[13,14] These characteristics are crucial in determining whether a biliary tract tumor is surgically resectable and thereby potentially curable. A tissue diagnosis is often obtained via endoscopic retrograde cholangiopancreatography (ERCP) and via biliary tract brushings. Resectable disease is defined by the absence of multifocal liver disease, lymph node metastasis beyond the porta hepatis, and distant metastasis. Resectability status is ultimately determined by a multidisciplinary team of experienced radiologists and surgeons. In patient's with hilar cholangiocarcinoma, biopsy should not be performed until resectability status and transplant candidacy have been determined because transperitoneal biopsy may preclude transplantation depending on institutions' protocols. In patients with unresectable disease, biopsies should be performed for tissue analysis and molecular profiling, including next-generation sequencing, to identify actionable mutations. In addition, positron emission tomography (PET) and diagnostic laparoscopy can help detect regional lymph node involvement and distant metastasis, although PET scans have a higher propensity for falsely detecting sites of disease that are not actually sites of metastasis.[15–17]
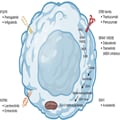
While chemotherapy has historically been the mainstay of treatment for patients with advanced cholangiocarcinoma, targeted molecular therapies are increasingly utilized in clinical practice due to newly identified alterations and the desire to reduce adverse effects associated with cytotoxic therapy (Figure 1). One analysis postulated that approximately 68% of patients with BTCs have an actionable mutation.[18] In this review, we examine the evidence supporting the utilization of targeted therapies for patients with advanced BTCs. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-22-93/rc).
Figure 1.
Key targets in biliary tract cancers. Created with BioRender.com. FGFR, fibroblast growth factor receptor; NTRK, neurotrophic tyrosine receptor kinase; RAS, rat sarcoma; BRAF, v-raf murine sarcoma viral oncogene homolog B1; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; IDH1, isocitrate dehydrogenase 1; ERB, epidermal growth factor.
Chin Clin Oncol. 2023;12(2):14 © 2023 AME Publishing Company