When reading the ECG of a patient presenting with cardiopulmonary complaints, the most important duty of acute care clinicians is to determine whether there is evidence of an acute coronary occlusion (ACO).
Patients with an ACO need immediate reperfusion therapy via cardiac catheterization with percutaneous coronary intervention or fibrinolysis. Traditional instruction has taught that patients with an ACO will demonstrate ST-segment elevation (STE) in contiguous leads on the ECG. These cases are referred to as ST-segment elevation myocardial infarction (STEMI).
Unfortunately, traditional guidance to look for STE has largely led to a dichotomy in how we have cared for patients emergently: Patients with STE in contiguous leads (presumed STEMI) have received immediate reperfusion therapy, and patients without STE in contiguous leads have simply received antiplatelet and antianginal therapy and, at best, nonemergent cardiac catheterization. The only widely accepted exception to this was when posterior STEMI was suspected. This type of STEMI tends to produce ST-segment depression in the right precordial leads; therefore, when this pattern is found, we have been taught to obtain posterior leads to verify the presence of posterior STEMI and initiate acute reperfusion therapy.
For the past two decades, however, studies have demonstrated that ACO does not always produce the classic STE in contiguous leads. In fact, these studies have suggested that 25% to 38% of patients with ACO will not demonstrate expected STE. These non-STE ACO patients are often deprived of the opportunity for immediate reperfusion therapy, resulting in potentially greater cardiac morbidity and mortality.
Despite this evidence, guidelines from the American College of Cardiology (ACC) continued to recommend immediate reperfusion therapy on the basis of the initial ECG only when STE in contiguous leads (or posterior STEMI) was found. But change has finally arrived.
The ACC convened a panel of experts to provide recommendations on an assortment of topics relating to the evaluation and management of patients presenting to the emergency department with chest pain. Most relevant to our discussion, the panel identified causes of non-STE ACO, or what they refer to as "STEMI equivalents." They reaffirmed the importance of identifying posterior STEMI and performing immediate reperfusion therapy in those patients. Most notably, they specifically indicated for the first time that patients with left bundle branch block (LBBB) patterns or right ventricular pacemakers who manifest Sgarbossa criteria or modified Sgarbossa criteria, and patients with de Winter sign, should undergo emergent coronary angiography. Three patterns are reviewed below.
Left Bundle Branch Block With Sgarbossa Criteria
In 1996, Sgarbossa published a set of ECG criteria that predicted the presence of acute MI in the presence of LBBB. These criteria consisted of any of the following on any single lead of the ECG:
A. Concordant ST-segment elevation of ≥ 1 mm in any lead (Figure 1).
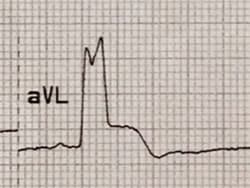
Figure 1.
B. Concordant ST-segment depression of ≥ 1 mm in any of leads V1-V3 (Figure 2).

Figure 2.
C. Discordant ST-segment elevation of ≥ 5 mm in any lead (Figure 3).

Figure 3.
The third criteria, criteria C, lacked specificity, so in 2012, Smith and colleagues published and later validated a modification of the third criteria, referred to as the modified Sgarbossa criteria. This modification was associated with significantly greater specificity for ACO:
Modified C. Discordant ST-segment elevation with ST/S ratio > 25% in any lead (Figure 4).

Figure 4.
Right Ventricular Pacer With Sgarbossa Criteria
Because patients with right ventricular pacers typically manifest ECG findings similar to those of patients with LBBB, Sgarbossa surmised that the criteria identifying acute MI in patients with LBBB would also identify MI in patients with right ventricular pacers. In 1996, she published a set of ECG criteria that did, in fact, predict the presence of MI in these patients:
A. Concordant ST-segment elevation of > 1 mm in any lead (see Figure 5).

Figure 5.
B. Concordant ST-segment depression of > 1 mm in any of leads V1-V6 (see Figure 6).

Figure 6.
C. Discordant ST-segment elevation of ≥ 5 mm in any lead (Figure 7).

Figure 7.
In 2021, Dodd and colleagues published a modification to these Sgarbossa criteria for pacers, and these were discussed in greater detail on Medscape last November. These modified criteria provided slight revisions of the second and third criteria:
A. Concordant ST-segment elevation of ≥ 1 mm in any lead.
Modified B. Concordant ST-segment depression of ≥ 1 mm in any of leads V1-V6.
Modified C. Discordant ST-segment elevation with ST/S ratio > 25% in any lead (Figure 8).

Figure 8.
de Winter Sign
In 2008, de Winter and colleagues identified an ECG pattern that predicted the presence of an acutely unstable critical lesion in the proximal left anterior descending artery in patients presenting with cardiopulmonary complaints (Figure 9). The pattern consisted of upsloping precordial ST-segment depressions rising into tall symmetric T waves, sometimes accompanied by 0.5 mm -1.0 mm of STE in lead aVR.

Figure 9.
The panel also recommended vigilance for the presence of hyperacute T waves (broad asymmetric peaked T waves), as these can be seen early in the presence of an ACO. However, they stopped short of recommending immediate reperfusion therapy for these patients, instead recommending serial ECGs to assess for progression to STEMI.
Final Thoughts
We have been discussing the Sgarbossa criteria, the modified Sgarbossa criteria, and the de Winter sign for quite a few years in conferences and on social media. However, this new consensus document is the first formal endorsement by the ACC that patients with these ECG findings warrant immediate reperfusion therapy. All acute care clinicians should now work to become expert in recognizing these findings and to incorporate these criteria into their hospital protocols for acute reperfusion therapy.
This publication also represents a major step forward in formally acknowledging that many cases of ACO do not manifest typical STE in contiguous leads. I am hopeful that in the coming months and years, our national guidelines will begin to recognize other STEMI equivalents as well.
Amal Mattu, MD, is a professor, vice chair of education, and co-director of the emergency cardiology fellowship in the Department of Emergency Medicine at the University of Maryland School of Medicine in Baltimore.
Follow Dr Mattu on Twitter.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Image credits:
Lead image: iStock/Getty images
Images 1-9: Amal Mattu, MD
Medscape Emergency Medicine © 2022 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Amal Mattu. STEMI Equivalents You've Got to Know - Medscape - Nov 14, 2022.












Comments