The lifesaving COVID-19 vaccine, as with all medications and vaccinations, is associated with adverse reactions, some vaccine-related and some not, some serious but mostly not. Yet those adverse reactions still stoke fear in unvaccinated and partially vaccinated persons and also pose challenges to the medical community. Aside from the expected flu-like symptoms reflective of the immune response itself, many common side effects are dermatologic.
The Most Common Reported Skin Reaction
According to an international dermatology registry, it was "erythema, swelling, tenderness, pain, induration, and pruritus within 7 days after injection."[1] Reactions tended to be more common in younger, mostly female patients. Similar reactions occurred in a small minority of persons in a delayed fashion, 8 days or more later. These were typically mild, large, local swelling of brief duration. Biopsies of these lesions in mRNA-vaccinated patients were consistent with a T-cell–mediated hypersensitivity reaction. Treatment was often deemed unnecessary, but some patients were treated with antihistamines and topical or oral corticosteroids.
Chronic Urticaria and Atopic Dermatitis
An abstract[2] presented at the 2022 American Academy of Allergy, Asthma, and Immunology annual meeting presented a small case series of both chronic urticaria (CU) and atopic dermatitis reactions to first and second doses of COVID mRNA vaccines. Whereas there were a few new-onset eczematous eruptions postvaccination, there were flares in patients with preexisting atopic dermatitis .
Only a minority of patients with preexisting CU (7 of 67) had a CU flare. Those seven patients had a significantly higher median anti-IgE receptor antibody titer compared with those who did not react to the COVID-19 vaccine. All of those patients either started omalizumab or increased their dose of this drug (an anti-IgE receptor–blocking antibody). The six patients with new-onset urticaria presented 1-19 days after vaccination, but one of the patients receiving a second booster reacted within 1 day. The authors concluded that as a non–life-threatening disorder, CU is not a contraindication to receiving additional COVID-19 vaccinations.
My Experience
Since the COVID-19 vaccines were first introduced, I have seen 63 patients with urticaria and angioedema. Of these, 10 had more severe chronic idiopathic urticaria that was being treated with the monoclonal anti-IgE antibody omalizumab, and none of these experienced a flare after COVID-19 vaccination. Among the other 53 patients, six presented with various exanthems after vaccination.
Patient 1: This 46-year-old woman with a history of Hashimoto thyroiditis and psoriasis had delayed hives 6 days after her first Pfizer vaccine. The protracted course was treated by her primary physician with high-dose oral steroids (60 mg for 4 days, then 40 mg for 4 days). On the final day of the oral steroid, she had a milder urticarial flare afterward. Her COVID-19 spike protein was positive after this, so the high-dose steroid did not suppress antibody production.
Patient 2: This 50-year-old woman had a history of dermatographia and cold urticaria and a family history of autoimmune disease (a brother with Graves disease). Her urticaria had been controlled with two 24-hour antihistamines until she received the Johnson & Johnson COVID vaccine, after which urticaria worsened but eventually receded without further intervention. She still proceeded with the second Pfizer dose as well as the Pfizer booster. She then contracted COVID and 1 month later had another flare. She was later diagnosed with Lyme disease.
Patient 3: This 54-year-old woman had a history of urticaria and angioedema in remission until 5 days after her first Pfizer vaccine. She was treated with prednisone, then high-dose fexofenadine, with good response.
Patient 4: A 36-year-old pregnant woman was evaluated toward the end of her second trimester for rash 12 days after receiving the Moderna booster. She had itching of the hands then the feet, which evolved into hand and foot swelling. Hand edema resolved spontaneously, but foot edema worsened. Examination showed mild hand edema with palmar erythema and dermatographia. She responded well to a single daily antihistamine and a brief low-dose tapering course of prednisone. Symptoms receded within 1 or 2 weeks. Three weeks later, she had COVID-19 but no rash.
Patient 5: A 53-year-old woman noted a diffuse exanthem 10 days after the first Moderna vaccine. Lesions were raised, responded slightly to antihistamines, and resolved within 2 days.
Patient 6: This 45-year-old woman with a history of psoriasis presented with a rash a few hours after her first vaccine (Moderna). About 8 hours, later she had knee pain, back pain, fever to 100.5 °F, dizziness, chills, and diarrhea. There was moderate erythema on the extensor surfaces of her wrists, knees, and ankles (Figure 1). A papular exanthem progressed somewhat without blistering and improved with topical steroids. Dizziness lasted for 2 weeks. Her antinuclear antibody titer was 1:80, and thyroid peroxidase was elevated. Before her second vaccine, we discussed her options, including oral steroid premedication. She elected to proceed with the Moderna vaccine, resulting in a similar reaction that again was managed with topical steroids. Surprisingly, she still decided to receive Moderna's product for her third vaccine (ie, her first booster). She again had a similar reaction yet is wavering about a switch to a different COVID-19 vaccine when the time comes. (For perspective, the patient had lost four loved ones to COVID and preferred the risk for these adverse events to the risk for disease.)
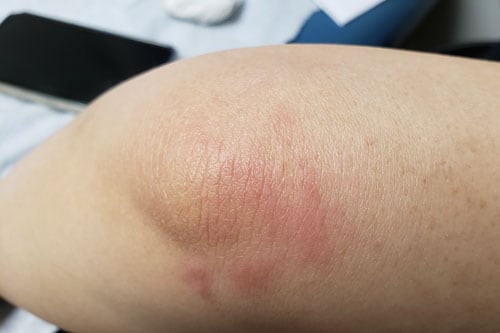
Figure 1. Focal urticaria, interestingly in the area extensor surface of an extremity joint, a common location of psoriatic eruptions.
Patient 7: A 25-year-old man with a long history of recurrent heartburn, sore throat, and nasopharyngitis presented with an evolving rash 9 days after his Moderna booster. He had had a sore throat with heartburn and had just tested negative for COVID-19 by PCR. He also had fever, myalgia, and fatigue. He had symmetric dusky urticarial lesions on wrists, antecubital fossa, axilla, hips, and back with scattered targetoid features (Figure 2). Lab studies were notable for a C-reactive protein level of 88 and slight neutrophilia. Eosinophil count was 100, and extensive rheumatology panel was negative. He was treated with prednisone 40 mg and a few days later had fatigue; hand, knuckle, and palmar swelling; and mild periorbital urticaria that receded slowly as the steroids were tapered over 4 weeks. Itching and dermatographia occurred, and fexofenadine 180 mg four times daily was added to the regimen.(Fexofenadine is approved for once-daily use, but allergists routinely prescribe higher doses for severe urticaria cases. This is a widespread practice and is also supported by the medical literature.) Six weeks after presentation, he was down to 5 mg prednisone daily and noted distal toe mottling and subcutaneous bruising concentrated near the toenails, which resolved without treatment. It is now 6 months later, and he has sporadic hives after exercise and dermatographia.
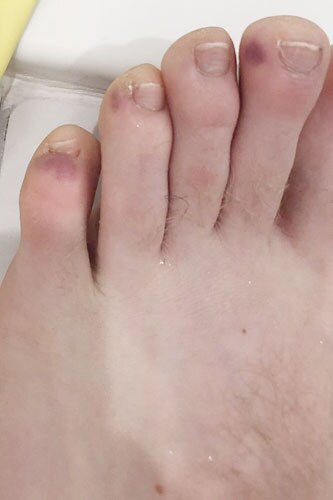
Figure 2. Periungual hematomas, suggestive of "COVID toes," with no indication of more significant vascular involvement.
Among my small sample of seven patients, six were female, two had prior urticaria or angioedema, and two had an autoimmune condition. One of the patients with preexisting urticaria also had a brother with Graves disease. Reactions to all three vaccine types were observed, and these occurred with the first, second, or booster doses.
Patient 7 had a moderate reaction requiring oral steroids. He had a sore throat that I believe was due to one of his recurrent episodes gastroesophageal reflux disease/sore throat episodes. In support of this is the absence of any other respiratory symptoms (nasal, chest, or anosmia). Despite the possibility of this having been infectious in etiology, I included his case here because he represents the sort of challenge one encounters in practice. The appearance of the toe eruption, which looked like "COVID toes," has also been reported in a patient vaccinated to COVID in France.[3]
Given the frequency of urticaria, angioedema, and various exanthemata, it's difficult to know whether these episodes were spontaneous or triggered by the vaccines. As I see it, the vaccine was unquestionably the cause in patient 6, who had three identical postvaccination reactions. Her case presented a number of quandaries: Is it safe to proceed again with that vaccine, or switch to another mRNA vaccine (Pfizer) or the adenovirus vector vaccine (Johnson & Johnson)? The literature offers no clear answers.
The second question was whether premedication with oral steroids might have attenuated the immune response to the vaccine, rendering it less effective. That did not happen with patient 1, whose course of oral steroids did not prevent the development of spike antibodies. Perhaps we can also extrapolate from studies of patients with asthma treated with 16 mg of methylprednisolone who had no diminution in response to the influenza vaccine despite some transient changes in immune function.[4]
In summary, the COVID-19 vaccine is associated with occasional worsening of preexisting urticaria or angioedema as well as de novo reactions after vaccination, but serious adverse reactions are quite rare. Only one patient in my practice definitively reacted to the vaccine (thrice!). Another patient had a moderate protracted exanthem requiring long-term oral steroids, and he remains mildly symptomatic 6 months later. Only the "COVID-toes" rash suggested a connection to the vaccine.
All in all, the body of evidence supports the safety of COVID-19 vaccinations even in patients with prior urticaria, angioedema, and atopic dermatitis. De novo reactions may occur in patients with a personal or family history of autoimmunity, but they do not seem to be more severe than others. It is my opinion that patients at risk for severe COVID-19 who have had mild reactions need not and should not avoid future vaccinations. Premedication in these patients can be considered on a case-by-case basis. Conversely, patients who are not at risk for severe COVID-19 and also have had more severe reactions should discuss their options with their physicians.
Gary J. Stadtmauer, MD, is an allergist-immunologist in New York City. His areas of clinical interest include asthma, eczema, chronic cough, and sinusitis. He has been a Medscape contributor since 2014.
Credits:
Image 1: Gary J. Stadtmauer, MD
Image 2: Gary J. Stadtmauer, MD
Medscape Allergy & Immunology © 2022 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Gary J. Stadtmauer. Diffuse Urticarial Eruptions After COVID-19 Vaccination - Medscape - Jul 18, 2022.











Comments